valence electrons of pt|2.9: Valence Electrons : Pilipinas Mar 23, 2023 You can find out your current zone by visiting our mapping application. What is the Purpose of the Rezoning Process? . City of Chilliwack ; Engineering; 8550 Young Rd Chilliwack, BC V2P 8A4; Phone: 604-793-2907; Click Here to .
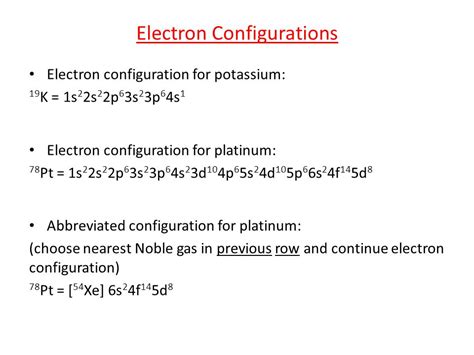
valence electrons of pt,The total number of electrons in platinum is seventy-eight. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons in platinum in specific rules in different orbits and orbitals is called the electron configurationof platinum. The electron configuration of . Tingnan ang higit pavalence electrons of pt 2.9: Valence Electrons Scientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there. The electronsof . Tingnan ang higit paAtomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable . Tingnan ang higit pa Mar 23, 2023 Updated on November 04, 2019. You may assume that the valences of the . Electron configuration of Platinum is [Xe] 4f14 5d9 6s1. Possible oxidation states are +2,4. Electron Configuration. The periodic table is a tabular display of the .Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the \(1s\) sublevel are called .

Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical . Determine the total number of valence electrons in the molecule or ion. Add together the valence electrons from each atom.Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2 s subshell and four in the 2 p .
Definition and Periodic Table. This entry was posted on March 3, 2021 by Anne Helmenstine (updated on January 3, 2023) A valence electron is an outer shell electron that can participate in a chemical . Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the \(1s\) .2.9: Valence Electrons 2. Find the electron configuration for the element you are examining. Once you know an element's electron configuration, finding .Platinum (Pt) is a rare and precious metal with many uses and properties. Learn about its history, abundance, physical and chemical characteristics, electron configuration, isotopes, health and safety aspects, and how it compares with other elements in the periodic table. Explore the interactive and dynamic features of SchoolMyKids website to enhance your .Contributors and Attributions. 3.10: Valence Electrons is shared under a CC BY-NC license and was authored, remixed, and/or curated by LibreTexts. Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s sublevel are called inner-shell electrons ..
Figure 15.4.3 15.4. 3: The ammonium ion. When drawing the Lewis structure of a polyatomic ion, the charge of the ion is reflected in the number of total valence electrons in the structure. In the case of the ammonium ion: 1 N 1 N atom = 5 = 5 valence electrons. 4H 4 H atoms = 4 × 1 = 4 = 4 × 1 = 4 valence electrons.We would like to show you a description here but the site won’t allow us.Protons, Neutrons, Electrons for Platinum (Pt, Pt4+) Platinum is a classified transition metal and its symbol is ‘Pt’. Platinum is the 78th element of the periodic table so its atomic number is 78. The atomic number of an element is equal to the number of protons and electrons in that element. Therefore, a platinum atom has seventy-eight .
sulfur. helium. potassium. aluminum. Solution. Sulfur (S) is located in Group VIA (Group 16), so it has 6 valence electrons. Helium (He) is located in Group VIIIA (Group 18). However, one atom only has two electrons, so it could never have more than 2 valence electrons. As noted above, helium is the only exception for the main group .
The valence electrons are part of the chemical reactions because they contain more energy compared to the electrons present in inner orbits. Meanwhile, the number of valence electrons present also helps us determine a specific element’s chemical properties, such as its valence or valency, the formation of bonds with other elements. As a gas or vapor, the halogens all had a pungent odor. After the development of quantum mechanics, it was shown that the halogens all had seven valence electrons, supporting their original placement into the same group on Mendeleev's periodic table. Figure 11.1.1 11.1. 1: Periodic table by Dmitri Mendeleev, 1871. Valence electrons are those electrons that reside in the outermost shell surrounding an atomic nucleus. Valence electrons are of crucial importance because they lend deep insight into an element’s chemical properties: whether it is electronegative or electropositive in nature, or they indicate the bond order of a chemical compound – the .Orbital diagram. Platinum electron configuration. Pt (Platinum) is an element with position number 78 in the periodic table. Located in the VI period. Melting point: 1772 ℃. Density: 21.45 g/cm 3 . The order of filling the orbitals with electrons in the Pt atom is an exception to the rule. Expected electronic configuration 1s2 2s2 2p6 3s2 3p6 . These are homework exercises to accompany Chapter 4 of the Furman University's LibreText for CHE 101 - Chemistry and Global Awareness. 4: Valence Electrons and Bonding. Valence electrons are outer shell electrons with an atom and can participate in the formation of chemical bonds. In single covalent bonds, typically .valence electrons of ptElectrons that are found in the outermost shell are generally known as valence electrons and the number of valence electrons determines the valency (or valence) of an atom. . The number of bonds that an atom .

Valence electrons are the electrons present in the outermost shell of an atom. You can easily determine the number of valence electrons an atom can have by looking at its Group in the periodic table. For example, atoms in Groups 1 and 2 have 1 and 2 valence electrons, respectively. Atoms in Groups 13 and 18 have 3 and 8 valence electrons . The valency of an element represents the number of bonds that an atom can form as part of a chemical compound. The atomic number of the element platinum in the periodic table is $78$ and its atomic mass is $195$. The electronic configuration of platinum is $[Xe]4{f^{14}}5{d^9}6{s^1}$ . The valency of platinum is $4$ or $2$ .The atomic number of platinum is 78, which means it has 78 electrons. Now it is possible to find the orbital notation of platinum very easily through electron configuration. That is, the orbital notation of platinum is 1s 2 2s 2 2p 6 3s 2 3p . 3.1: Valence Electrons is shared under a CC BY-NC license and was authored, remixed, and/or curated by LibreTexts. Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s sublevel are called inner-shell electrons ..
valence electrons of pt|2.9: Valence Electrons
PH0 · What Are Valence Electrons? Definition and Periodic Table
PH1 · Valences of the Elements Chemistry Table
PH2 · Valence electrons (video)
PH3 · Valence Electrons Chart for All Elements
PH4 · Valence Electrons
PH5 · Platinum
PH6 · Complete Electron Configuration for Platinum (Pt)
PH7 · 3.10: Valence Electrons
PH8 · 2.9: Valence Electrons
PH9 · 15.4: Lewis Structures: Counting Valence Electrons
PH10 · 10.6: Valence Electrons